A new ALS drug is extending patients’ lives by months. It’s giving hope for those facing such a ‘brutal illness.’
Published in USA Today March 3, 2021, written by Jayne O'Donnel
“Every day is a great day.” That’s what Nancy Poon, a lifelong runner, tells herself about a life now spent in a wheelchair. She was diagnosed in December 2018 with amyotrophic lateral sclerosis, also known as Lou Gehrig’s disease.
She enjoys the swims she can still do two weeks a month when she’s not undergoing drug infusions – even though she can no longer manage a flutter kick and must rely on her arms to pull her through the water. She’s even glad she can help prepare meals and deal with dirty clothes.
“Whoever thought you’d love to do laundry? But it’s something I can still do,” said Poon, 64, of Wakefield, Rhode Island.
The personal pep talk is Poon’s way of staying positive in the face of a disease she calls “worse than any disease you can possibly have. It’s a slow, slow demise where you lose parts of yourself.”
Poon and the 15,000 or so other ALS patients in the U.S. have more to feel good about this year. Patients and researchers cite three positive developments:
- A new drug – developed in part with money from the 2014 Ice Bucket Challenge – increased the average survival of patients in a clinical trial by seven and half months.
- A first-ever clinical trial is testing multiple ALS treatments at once.
- Record levels of federal research funding are planned this year.
After decades of clinical trials for treatments that ended in dashed dreams, Poon and other ALS patients are hopeful that Amylyx’s AMX0035, which got a $2.2 million grant from the $111 million Ice Bucket Challenge, will be approved by the Food and Drug Administration.
That’s the drug that added seven months to patients’ lives – significant considering patients’ life expectancy is between two and five years.
Findings for AMX0035’s Phase 2 trial, which is when a drug is tested for efficacy and side effects, were so promising that the ALS community is pushing the FDA to fast-track its approval. The ALS Association asked the FDA to skip a Phase 3 trial – believed to be the only time the association has done that, said Neil Thakur, the ALS Association’s chief mission officer.
But like everything else with ALS treatment, it’s a long shot. Despite regular pressure, the FDA rarely approves drugs without completing a large, Phase 3 trial.
Nancy Poon, who has ALS, can no longer do the athletics she loved, but enjoys doing household chores – because she still can.” “Robert Deutsch, USA TODAY
In late January, the Margolis Center for Health Policy at Duke University held a two-day public meeting with FDA officials and other experts to discuss ways to improve research and make ALS clinical trials more innovative.
Dr. Mark McClellan, a former FDA commissioner who heads the Duke center, said the meeting focused on the FDA’s commitment to do more research to get evidence more quickly, including directly from patients and caregivers.”It gave me hope,” McClellan said. “This effort should help bring more promising treatments forward into good clinical studies.”
Still, McClellan cautioned, “there’s no guarantee really effective treatment is right around the corner.”
ALS patients push to fast-track drug approval
ALS is a priority at the FDA, said Dr. Janet Woodcock, director of the FDA’s Center for Drug Evaluation and Research and now acting FDA commissioner.
When the FDA receives a new drug application to treat advanced cancers or other diseases like ALS that progress rapidly, Woodcock told USA TODAY, the agency can approve it quickly – depending on the results, safety and the company’s ability to manufacture it.
She said that can be done if there is “a huge unmet need and a compelling result.” She wouldn’t comment on specific drugs.
In a statement, Amylyx co-CEOs Joshua Cohen and Justin Klee said the drugmaker is “in discussions with FDA about paths to approval and expanded access.”
“We understand that patients with ALS have no time to wait,” they said.
‘The value of good, solid evidence’
Despite the pressure to speed approval, there’s a reason drugs must undergo large Phase 3 trials: They find problems that earlier, smaller trials don’t.
A 2017 FDA report looked at 22 drugs that had very different results in Phase 3 than Phase 2. It concluded Phase 3 trials “can generate critical evidence,” noting they have found Phase 3 failures in safety and efficacy even for drugs approved for other conditions.
Dr. Joel Lexchin, a health policy professor emeritus at Toronto’s York University, has treated ALS patients and others with rare diseases as an emergency room doctor. He said he has “a lot of sympathy for these people, but at the same time, I understand the value of good, solid evidence before rushing into using new drugs.”
He cites the high-dose chemotherapy and stem cell transplants given to more than 40,000 women in the 1990s based on preliminary evidence it could cure their metastatic breast cancer. The treatment turned out to have no benefit, and “a lot of women suffered in the process,” he said.
“We hear a lot about potential benefits in the early stages of clinical development, but we don’t hear so much about the harms they can also show,” said Lexchin, who co-authored a 2016 report in the British medical journal BMJ cautioning against U.S. and European moves to approve drugs with less data.
ALS patients have gotten used to being disappointed. Brainstorm’s NurOwn, which uses a patient’s stem cells, had promising Phase 2 results, butfurther research showedthe treatment was found to slow progression in about a third of patients – not a statistically significant difference from the placebo group.
Last week, Brainstorm announced the FDA had told the company its initial review of NurOwn’s data from Phase 3 wasn’t enough to approve it.
23-year-old soccer player diagnosed
About 5,000 people are diagnosed with ALS every year. Most people develop ALS between the ages of 40 and 70, with 55 the average age at diagnosis. There’s no cure. And the disease is ruthless.
Ryan May, 23, of Huntsville, Utah, started experiencing symptoms while he was on his honeymoon in September 2018. It started with twitching in his legs that eventually spread to his whole body. Within months, May, a former soccer player, was diagnosed with ALS, and enrolled in the NurOwn trial.
Ryan and Rachel May are shown at their September 2018 wedding. Ryan, then 21, started experiencing ALS symptoms on his honeymoon. He’s now using a feeding tube. Rachel Stephens
May is the youngest patient neurologist Dr. Namita Goyal has ever diagnosed. He is in a wheelchair and uses a feeding tube, with limited use of his arms and no use of his legs.
Unwilling to accept the rapid progression from “symptom onset to death,” Goyal said she chose to specialize in ALS treatments.
“It was something that spoke to my heart right away,” she said. “To go through that with one patient, let alone hundreds and hundreds, breaks my heart.”
Goyal has enrolled patients in about 30 clinical trials over the years. After starting at Massachusetts General Hospital, she is now at University of California, Irvine, where she moved to enable greater access to clinical trials on the West Coast.
She recalled thinking, “There are so many scientific advancements in medicine, how is it possible that a terminal disease has no effective therapy to stop or slow it down tremendously?”
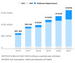
Federal funding expected to rise for ALS research
Neil Thakur, chief mission officer of the ALS Association, said the planned increase in federal funding for ALS research is among the reasons this year holds so much hope for patients and researchers.
More research into ALS treatments
The pace of ALS research has picked up. Thakur estimates there are about 80 current clinical trials for ALS treatments around the globe.
That’s about the same number of Phase 2 or 3 ALS trials completed, terminated, or suspended from 2007 to 2018, Goyal calculated in a study in the journal Muscle & Nerve in January 2020. In that time, she said, just one treatment was approved by the FDA to slow the progression of ALS: Edaravone, in 2017.
Stacy Lindborg, Brainstorm’s executive vice president and head of global clinical research, has a personal connection to ALS. She watched as her beloved uncle, Gerry Gunnin, suffered and died of it in 2003.
“It’s a brutal illness. It’s relentlessly progressive,” she said. “Your mental capacity is completely intact, but your body dies around you. … Swallowing, breathing and turning over in bed is gradually taken from you, and you are completely aware of everything that’s going on.”
Garry Gunnin, his niece Stacy Lindborg and wife Barbara Gunnin before Gerry’s death from ALS in 2003. Lindborg is now executive vice president and head of global research for Brianstorm Cell Therapeutics. Photo by Stacy R. Lindborg
One European country a year in retirement
Poon read neurosurgeon Paul Kalanithi’s ALS memoir, “When Breath Becomes Air,” after she retired in June 2017 following a career as an elementary school teacher in Connecticut and Rhode Island.
Almost immediately after retiring, the woman who had completed a marathon and several sprint triathlons started tripping while running.
Poon tried to get into the NurOwn clinical trial, but the disease was progressing too slowly for her to qualify. She’s now in Massachusetts General’s Healey ALS Platform trial that includes four drugs, reducing the chance patients will be on a placebo.
Nancy Poon, who has ALS, is shown in her Rhode Island kitchen with husband Alan Poon.
Gone are her retirement plans with husband to rent a home for two to three months each year in a different country. She saw Spain and France in the summer of 2019 in a wheelchair, took a chartered sailboat trip to the Caribbean soon after her diagnosis, and became an active member of the ALS community in Rhode Island.
“As horrible as this disease is, you realize how much you’re loved by family and friends and how many support you,” Poon said. “Not many people get to experience that in their lifetime.”
Woodcock, who credits the ALS community for its work with the FDA and the Ice Bucket Challenge, also sees hope.
“We’re paying a lot of attention to ALS because there is hope on the horizon for neurodegenerative diseases,” said Woodcock, citing approval of two drugs to treat spinal muscular atrophy in infants and children. “There’s been a pretty long dry spell.”
Poon’s 85 friends and family members were the largest group at ALS Rhode Island’s gala in 2019, and they’ve raised tens of thousands to support ALS research.